REBYOTA has confirmed >90% coverage for commercially and government-insured patients.* Learn More
REBYOTA is the first and only single-dose microbiota-based live biotherapeutic approved to prevent recurrence of C. difficile infection starting at first recurrence1,2,a
aIn the pivotal phase 3 trial, 32.8% of patients were treated at first recurrence of C. diff infection (CDI) following antibiotic treatment of CDI.1
REBYOTA WAS STUDIED IN ~1000 PATIENTS ACROSS 5 CLINICAL TRIALS1,3-7,b
| STUDY DESIGN CRITERIAb |
PUNCHTM CD3 (Phase 2) NCT01925417 |
PUNCHTM CD24 (Phase 2b) NCT02299570 |
PUNCHTM Open-Label5 (Phase 2) NCT02589847 |
PUNCHTM CD36 (Phase 3) NCT03244644 |
PUNCHTM CD3-OLS7 (Phase 3) NCT03931941 |
|---|---|---|---|---|---|
| Total Patients Enrolled | N=40 | N=150 | N=272 | N=320 | N=793 |
| CONSISTENT Population Patients with recurrent CDI (rCDI) |
|||||
| CONSISTENT Composition Composition of live fecal microbes |
|||||
| CONSISTENT Endpoint Absence of CDI diarrhea within 8 weeks |
|||||
| Study Type | Open-label | Randomized, controlled | Open-label | Randomized, controlled | Open-label |
| Follow-Up Duration | 6 months | 2 years | 2 years | 6 months | 6 months |
- Participants across 5 trials were not excluded if they had a history of food allergies1
bIn PUNCH™ CD2 and PUNCH™ CD3, participants were excluded if they had a known history of refractory CDI, IBD, IBS, chronic diarrhea, celiac disease, colostomy, active colitis, required antibiotic therapy for another condition, or had a history of fecal matter transplant.1,3,6 PUNCH™ CD3-OLS did not exclude individuals with celiac disease, IBD, IBS, and chronic diarrhea.7
PIVOTAL PUNCH™ CD3 TRIAL
Enrolled 320 patients with documented recurrent CDI (≥1 recurrence after a primary episode of CDIc or ≥2 episodes of severe CDI resulting in hospitalization within the last year).1,6
Investigator’s choice: Diagnostic test(s) used and selection of antibiotic to address active infection.6,d
Polymerase chain reaction (PCR) was utilized in >70% of PUNCH™ CD3 participants to diagnose qualified CDI.6
No bowel prep/laxatives: Patients did not receive bowel prep or laxatives prior to REBYOTA® treatment.6
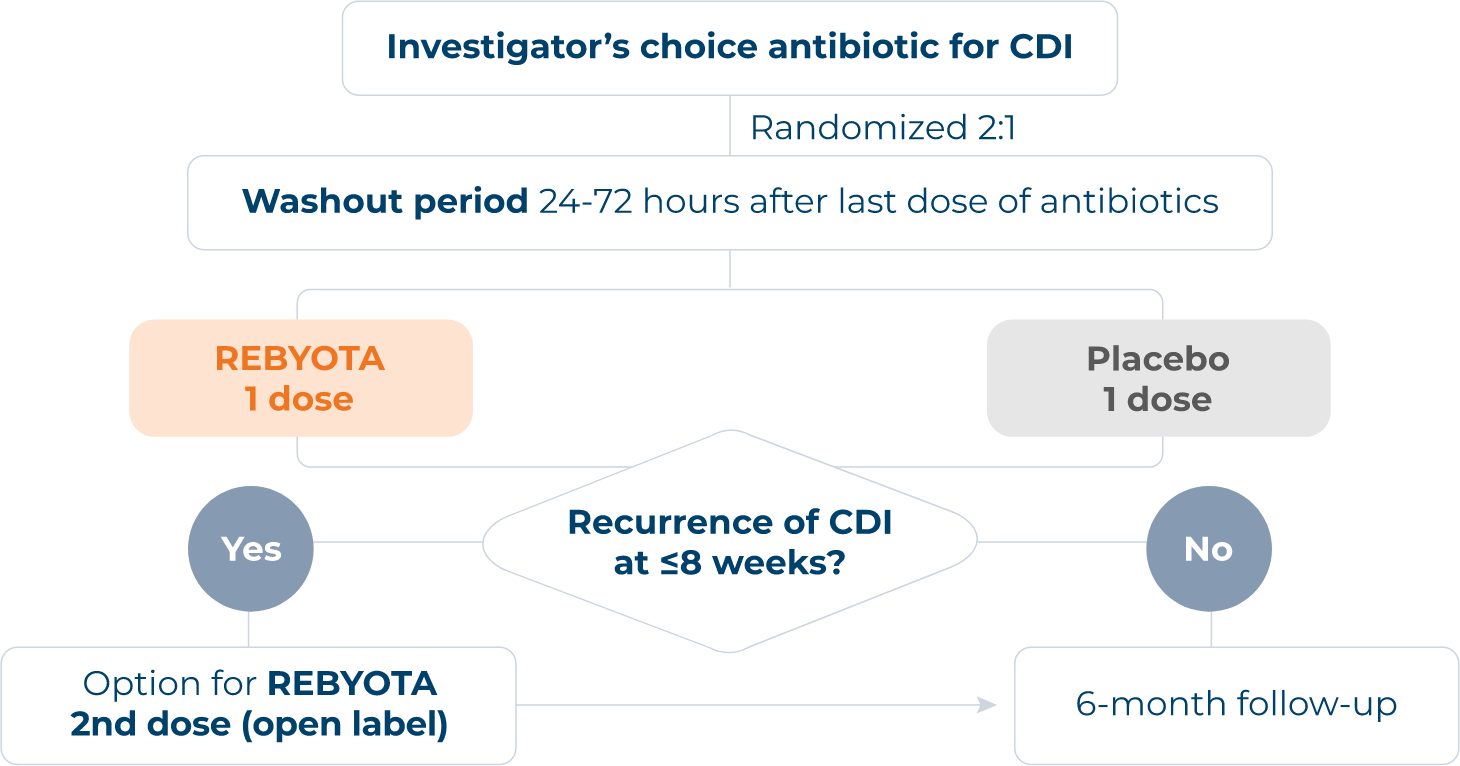
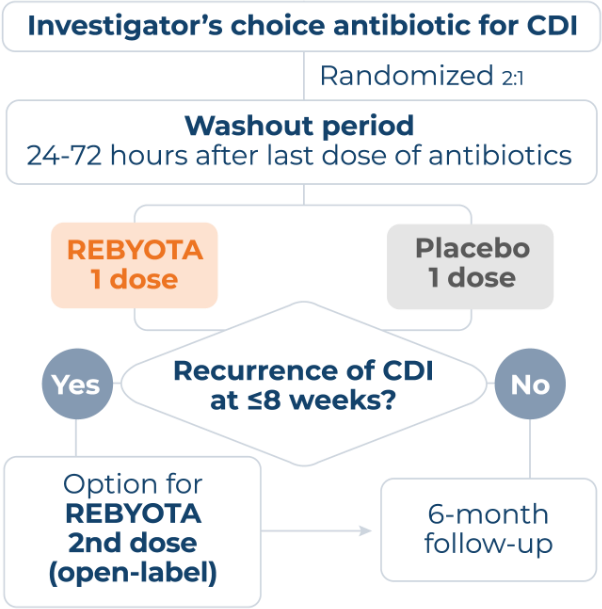
cPatients also must have completed >1 round of standard of care (SoC) antibiotic therapy.6
dCDI diagnosis confirmed 30 days before study enrollment via PCR, enzyme immunoassay, glutamate dehydrogenase, toxin A or B presence, or other CDI diagnostic test. Antibiotics (vancomycin, vancomycin in combination, fidaxomicin, or other) administered ≥10 consecutive days before washout.6
REBYOTA PREVENTED RECURRENCE OF CDI AT 8 WEEKS AND SUSTAINED RESPONSE THROUGH 6 MONTHS
In the pivotal PUNCH™ CD3 trial,
-
REBYOTA demonstrated a statistically significant
70.6% (126/177)
TREATMENT SUCCESS
RATEe AT 8 WEEKS
Placebo was 57.5% (53/85).1,6 -
Of those patients who saw success,
92.1% (116/126)
HAD A SUSTAINED RESPONSE
THROUGH 6 MONTHS
Placebo was 90.6% (48/53).1,6
Patients achieved treatment success6 regardless of…
Number of prior CDI episodesf • Agef • Sex • Race

Post hoc analysis from PUNCHTM CD3
-
Of 32.8% treated at FIRST recurrence of CDI1,8
79.2% (42/53)
OF PARTICIPANTS IN THE REBYOTA
GROUP HAD NO RECURRENCE
vs 60.6% (20/33) of those on placebo at 8 weeks9,g
REBYOTA SAFETY
FOLLOW-UP RANGED FROM 6 MONTHS TO 2 YEARS
Across the 5 trials in the clinical program, 978 patients had at least 1 dose of REBYOTA.1
In the PUNCH™ CD3 trial…
Adverse reactions reported by ≥3% of REBYOTA recipients (and at a rate greater than that reported by placebo recipients) within 8 weeks after receipt of REBYOTA or placebo.1,6,h
| ADVERSE REACTIONS, n (%)1 | REBYOTA n=180 |
Placebo n=87 |
|---|---|---|
| Abdominal pain | 16 (8.9%) | 6 (6.9%) |
| Diarrhea | 13 (7.2%) | 3 (3.4%) |
| Abdominal distension | 7 (3.9%) | 2 (2.3%) |
| Flatulence | 6 (3.3%) | 0 |
| Nausea | 6 (3.3%) | 1 (1.1%) |
Most adverse reactions occurred during the first 2 weeks after administration1
- Proportion of patients with adverse reactions declined in subsequent 2-week intervals thereafter
- Beyond 2 weeks after administration only a few single events were reported
Most adverse drug reactions were mild to moderate in severity1
- No life-threatening adverse reaction related to REBYOTA was reported
hAdverse reactions were defined as solicited and unsolicited adverse events that were assessed as definitely, possibly, or probably related to administration by the study investigator and that occurred within 8 weeks after blinded REBYOTA or placebo administration or until recurrence of CDI.1
Access Rebyota
Access information and support tools for REBYOTA including coding, billing, coverage, reimbursement, and financial assistance.
Ordering Instructions
There are multiple ways to order REBYOTA: through major specialty distributors, REBYOTA @ Home, or specialty pharmacies.
Sign up to receive information
and updates on REBYOTA
IMPORTANT SAFETY INFORMATION
INDICATION
REBYOTA (fecal microbiota, live – jslm) is indicated for the prevention of recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older, following antibiotic treatment for recurrent CDI.
Limitation of Use
REBYOTA is not indicated for treatment of CDI.
IMPORTANT SAFETY INFORMATION
Contraindications
Do not administer REBYOTA to individuals with a history of a severe allergic reaction (eg, anaphylaxis) to any of the known product components.
Warnings and Precautions
Transmissible infectious agents
Because REBYOTA is manufactured from human fecal matter, it may carry a risk of transmitting infectious agents. Any infection suspected by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Ferring Pharmaceuticals Inc.
Management of acute allergic reactions
Appropriate medical treatment must be immediately available in the event an acute anaphylactic reaction occurs following administration of REBYOTA.
Potential presence of food allergens
REBYOTA is manufactured from human fecal material and may contain food allergens. The potential for REBYOTA to cause adverse reactions due to food allergens is unknown.
Adverse Reactions
The most commonly reported (≥3%) adverse reactions occurring in adults following a single dose of REBYOTA were abdominal pain (8.9%), diarrhea (7.2%), abdominal distention (3.9%), flatulence (3.3%), and nausea (3.3%).
Use in Specific Populations
Pediatric Use
Safety and efficacy of REBYOTA in patients below 18 years of age have not been established.
Geriatric Use
Of the 978 adults who received REBYOTA, 48.8% were 65 years of age and over (n=477), and 25.7% were 75 years of age and over (n=251). Data from clinical studies of REBYOTA are not sufficient to determine if adults 65 years of age and older respond differently than younger adults.
You are encouraged to report negative side effects of prescription drugs to FDA. Visit www.FDA.gov/medwatch, or call
1-800-332-1088.
Please click here for full Prescribing Information.
References
- REBYOTA. Prescribing information. Parsippany, NJ: Ferring Pharmaceuticals Inc; 2022.
- US Food and Drug Administration. FDA Approves First Fecal Microbiota Product. Accessed December 1, 2022. https://www.ferring.com/ferring-receives-u-s-fda-approval-for-rebyota-fecal-microbiota-live-jslm-a-novel-first-in-class-microbiota-based-live-biotherapeutic/
- Orenstein R, Dubberke E, Hardi R, et al. Safety and durability of RBX2660 (microbiota suspension) for recurrent Clostridium difficile infection: results of the PUNCH CD study. Clin Infect Dis. 2016;62(5):596-602. doi:10.1093/cid/civ938.
- Dubberke ER, Lee CH, Orenstein R, Khanna S, Hecht G, Gerding DN. Results from a randomized, placebo-controlled clinical trial of a RBX2660-a microbiota-based drug for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018;67(8):1198-1204. doi:10.1093/cid/ciy259.
- Orenstein R, Dubberke ER, Khanna S, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect Dis. 2022;22(1):245. doi:10.1186/s12879-022-07256-y.
- Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527-1538. doi:10.1007/s40265-022-01797-x.
- Feuerstadt P, Chopra T, Knapple W, et al. PUNCH CD3-OLS: a phase 3 prospective observational cohort study to evaluate the safety and efficacy of fecal microbiota, live-jslm (REBYOTA) in adults with recurrent Clostridioides difficile infection. Clin Infect Dis. Published online August 24, 2024. doi:10.1093/cid/ciae437.
- Khanna S, Tillotson G, Ando M, et al. Efficacy and safety of RBX2660 in patients after first recurrence of Clostridioides difficile infection—results from a randomized, placebo-controlled, phase 3 study. Oral presentation at: American College of Gastroenterology Annual Meeting: October 21-26, 2022; Charlotte, NC.
- Feuerstadt P, Allegretti JR, Dubberke ER, et al. Efficacy and health-related quality of life impact of fecal microbiota, live-jslm: a post hoc analysis of PUNCH CD3 patients at first recurrence of Clostridioides difficile infection. Infect Dis Ther. 2024;13(1):221-236. doi: 10.1007/s40121-023-00907-w.



