REBYOTA has confirmed >90% coverage for commercially and government-insured patients.* Learn More
When the gut microbiome is in ruin,
Redefine Prevention Of
Recurrent c. difficile infection
and restore hope with REBYOTA®
DEDICATED J-CODE (J1440)
REBYOTA is the first and only single-dose microbiota-based live biotherapeutic approved to prevent recurrence of C. difficile infection starting at first recurrence1,2,a
aIn the pivotal phase 3 trial, 32.8% of patients were treated at first recurrence of C. diff infection (CDI) following antibiotic treatment of CDI.1
3 WAYS TO ORDER REBYOTA
There are multiple ways to obtain REBYOTA for your patients.* There may be payer-dependent requirements—running a benefits verification is the best way to confirm.
*Terms and conditions may apply.
-
Specialty Distributors (SD)
Order through an SD by setting up an account and following their application process.
-
REBYOTA @ Home
Offer your patients in-home administration with REBYOTA with consideration to location constraints, physical limitations, or administrative obstacles.
-
Specialty Pharmacy (SP)
Pending a patient benefits verification, you can order through an SP. You will work with the SP to handle all logistics.
REDEFINE DELIVERY OF LIVE MICROBIOTA IN ONE DOSE, ONE VISIT
Efficient delivery: REBYOTA is a pre-packaged 150-mL suspension that is rectally administered in any site of care by a healthcare professional.1
- No bowel prep/laxatives, fasting, anesthesia, or colonoscopy required3
- Conveniently order REBYOTA on-demand
- After administration, keep patient in position for up to 15 minutes to minimize possible cramping after which they can go home1
PREVENT RECURRENT C. DIFF WITH REBYOTA
ONE DOSE ADMINISTERED DIRECTLY WHERE
IT IS NEEDED—THE GUT MICROBIOME
One dose of REBYOTA provides trillions of essential live fecal microbes to restore a healthy gut microbiome and help prevent a C. diff recurrence.1,4

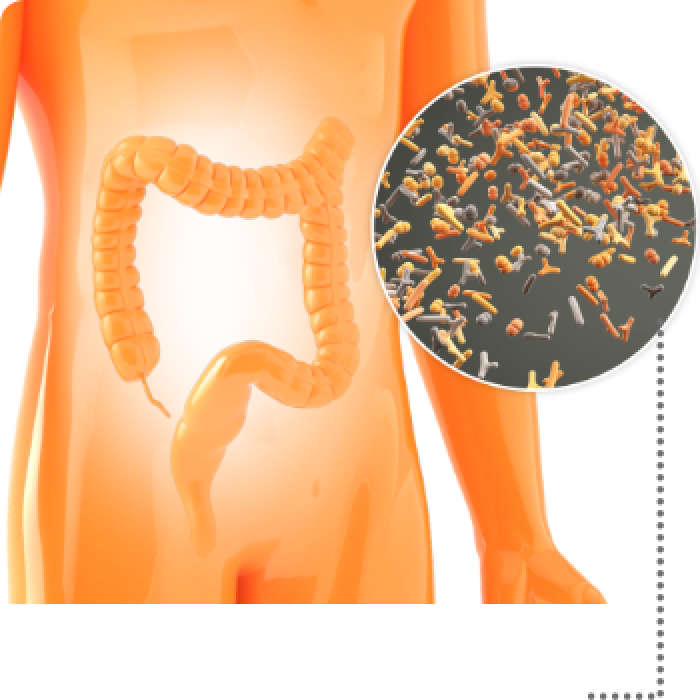
Loss of essential Bacteroidetes during dysbiosis is part of a dramatic, detrimental shift in the composition of the gut microbiome.4
Bacteroidetes are essential to maintaining biodiversity and human gut health, which leads to4:
- Improved immune function
- Resistance to C. diff colonization
One dose of REBYOTA provides:
15 BILLION TO
7.5 TRILLION
colony-forming units (CFU) of live fecal microbes and
>15 MILLION
CFU of essential Bacteroides1
of recurrent C. diff patients may experience a subsequent recurrence. REBYOTA is an important preventative measure to help stop C. diff from coming back.5,6
Standardized manufacturing process for an FDA-approved product7
-

REBYOTA is the filtrate generated by a proprietary manufacturing process adhering to good manufacturing practices (GMP)1
-
Consistent, rigorous health screening of donors and pathogen testing of stool donations for product safety3
-
Each dose is comprised of a donor’s single donation for ease of traceability—donations are not pooled7
-
Fecal microbes including live nonspore-forming Bacteroides are cryogenically preserved until ready for use8
REBYOTA—FOR ALL YOUR APPROPRIATE PATIENTS TO PREVENT rCDI
In a Ferring-sponsored survey of 100 REBYOTA-naive patients,
87%
of patients were accepting of rectal administration9,a
- FREDA, 70s
- MELISSA, 40s
- PATRICIA, 30s
FREDA, 70s

“I just knew that if I had C. diff again,
I wasn’t going to survive. But my C. diff didn’t
come back after I had REBYOTA.”
FREDA, age 70s
Actual PUNCH™ CD3 Trial Patient. Freda was compensated for her time. Individual results may vary.
Freda loves traveling to visit family, spending time with her husband on their farm, and caring for their bees and chickens.
She was hospitalized for severe
CDI symptoms—several days after she received antibiotics for a toothache.
After 4 severe recurrences, she and her husband feared for her life.
Most common adverse reactions include abdominal pain (8.9%), diarrhea (7.2%), abdominal distension (3.9%), flatulence (3.3%), and nausea (3.3%).1
C. difficile presentation and recurrences
- Experienced 4 recurrences in 6 months
- 6 months of diarrhea, 25-lb weight loss, weakness, and exhaustion
Freda’s journey
- Admitted for 5 days and given antibiotics after initial CDI diagnosis
- Received antibiotics after each subsequent recurrence. Symptoms would improve but kept returning
- Isolated from husband
- Enrolled in the PUNCH™ CD3 trial and received REBYOTA within 72 hours of completing antibiotics
- No recurrences since administration in 2019
“I got a manicure and went shopping!”
Individual results may vary.
Most common adverse reactions include abdominal pain (8.9%), diarrhea (7.2%), abdominal distension (3.9%), flatulence (3.3%), and nausea (3.3%).1
MELISSA, 40s

“Had REBYOTA been available sooner,
I would have asked for it.”
MELISSA, age 40s
Actual PUNCH™ CD3 Trial Patient. Melissa was compensated for her time. Individual results may vary.
Melissa lives a busy life, balancing marriage, a career, pets, creative writing, and being social—all while staying active and healthy.
However, her life shifted drastically when she was diagnosed with CDI.
Chronic fatigue and nutritional deficiencies became her new normal.
Most common adverse reactions include abdominal pain (8.9%), diarrhea (7.2%), abdominal distension (3.9%), flatulence (3.3%), and nausea (3.3%).1
C. difficile presentation and recurrences
- First recurrence occurred within days of completing an initial antibiotic course
- Experienced 5 recurrences in 11 months
- Had fatigue and nutritional deficiencies
Melissa’s journey
- Prescribed 10-day course of antibiotics after initial CDI diagnosis
- Received antibiotics after each subsequent recurrence. Symptoms would improve but kept returning
- Isolated from family
- Enrolled in the PUNCH™ CD3 trial and received REBYOTA within 72 hours of completing antibiotics
- No recurrences since administration in 2019
“I made dinner for the family for the first time in months.”
Individual results may vary.
Most common adverse reactions include abdominal pain (8.9%), diarrhea (7.2%), abdominal distension (3.9%), flatulence (3.3%), and nausea (3.3%).1
PATRICIA, 30s

“For me, it was one dose and one visit,
and that was it.”
PATRICIA, age 30s
Actual PUNCH™ CD3 Trial Patient. Patricia was compensated for her time. Individual results may vary.
Patricia is a doting, working mother of two, who loves activities like playtime and tea parties with her kids.
After a wave of severe symptoms disrupted her daily routine, she was diagnosed with CDI.
When it recurred, she was isolated inside, unable to play with her children, and ashamed to talk about her symptoms.
Most common adverse reactions include abdominal pain (8.9%), diarrhea (7.2%), abdominal distension (3.9%), flatulence (3.3%), and nausea (3.3%).1
C. difficile presentation and recurrences
- Experienced 3 recurrences in 3 months
- Diarrhea more than 20 times per day; weakness
Patricia’s journey
- Prescribed antibiotic after stool sample tested positive for CDI
- Primary care physician prescribed different antibiotics for each recurrence
- Isolated from family
- Enrolled in the PUNCH™ CD3 trial within days of completing antibiotics
- No recurrences since administration in 2019
“I was finally able to tuck my girls into bed again.
I read them a story and had lots of snuggles.”
Individual results may vary.
Most common adverse reactions include abdominal pain (8.9%), diarrhea (7.2%), abdominal distension (3.9%), flatulence (3.3%), and nausea (3.3%).1
Rebyota Clinical Data
Explore clinical data evaluating the efficacy and safety of REBYOTA for the prevention of recurrent CDI.
Rebyota Administration
Learn how to administer REBYOTA—one dose, one complete microbiome-based biotherapeutic.
Sign up to receive information
and updates on REBYOTA
IMPORTANT SAFETY INFORMATION
INDICATION
REBYOTA (fecal microbiota, live – jslm) is indicated for the prevention of recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older, following antibiotic treatment for recurrent CDI.
Limitation of Use
REBYOTA is not indicated for treatment of CDI.
IMPORTANT SAFETY INFORMATION
Contraindications
Do not administer REBYOTA to individuals with a history of a severe allergic reaction (eg, anaphylaxis) to any of the known product components.
Warnings and Precautions
Transmissible infectious agents
Because REBYOTA is manufactured from human fecal matter, it may carry a risk of transmitting infectious agents. Any infection suspected by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Ferring Pharmaceuticals Inc.
Management of acute allergic reactions
Appropriate medical treatment must be immediately available in the event an acute anaphylactic reaction occurs following administration of REBYOTA.
Potential presence of food allergens
REBYOTA is manufactured from human fecal material and may contain food allergens. The potential for REBYOTA to cause adverse reactions due to food allergens is unknown.
Adverse Reactions
The most commonly reported (≥3%) adverse reactions occurring in adults following a single dose of REBYOTA were abdominal pain (8.9%), diarrhea (7.2%), abdominal distention (3.9%), flatulence (3.3%), and nausea (3.3%).
Use in Specific Populations
Pediatric Use
Safety and efficacy of REBYOTA in patients below 18 years of age have not been established.
Geriatric Use
Of the 978 adults who received REBYOTA, 48.8% were 65 years of age and over (n=477), and 25.7% were 75 years of age and over (n=251). Data from clinical studies of REBYOTA are not sufficient to determine if adults 65 years of age and older respond differently than younger adults.
You are encouraged to report negative side effects of prescription drugs to FDA. Visit www.FDA.gov/medwatch, or call
1-800-332-1088.
Please click here for full Prescribing Information.
References
- Rebyota. Prescribing Information. Parsippany, NJ: Ferring Pharmaceuticals Inc; 2022.
- US Food and Drug Administration. FDA Approves First Fecal Microbiota Product. Accessed December 1, 2022. https://www.ferring.com/ferring-receives-u-s-fda-approval-for-rebyota-fecal-microbiota-live-jslm-a-novel-first-in-class-microbiota-based-live-biotherapeutic/ .
- Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527-1538. doi:10.1007/s40265-022-01797-x.
- Shin J, MacKenzie T, Tillotson GS. Bacteroidetes: The Jekyll and Hyde of the human gut microbiome. Pharmacy Times. Accessed November 19, 2022. https:// www.pharmacytimes.com/view/bacteroidetes-the-jekyll-and-hyde-of-the-human-gut-microbiome
- Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
- Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020.
- Ray A, Jones C. Does the donor matter? Donor vs patient effects in the outcome of a next-generation microbiota-based drug trial for recurrent Clostridium difficile infection. Future Microbiol. 2016;11(5):611-616. doi:10.2217/fmb.16.10.
- Papanicolas LE, Choo JM, Wang Y, et al. Bacterial viability in faecal transplants: which bacteria survive? EBioMedicine. 2019;41:s509-516. doi:10.1016/ j.ebiom.2019.02.023.
- Data on file. (pending publication).

